In-Silico Clinical Trials Market to hit $6.39B by 2033 | Key Growth Drivers & Outlook 2025
Next-Gen Drug Development Rises | In-Silico Trials Market to Reach $6.39B by 2033 with Strong United States. Leadership
U.S. Drives Surge in In-Silico Clinical Trials | Global Market Set to Hit $6.39B by 2033 with Rapid Digital R&D Adoption”
AUSTIN, TX, UNITED STATES, November 18, 2025 /EINPresswire.com/ -- Market Overview— DataM Intelligence 4Market Research LLP
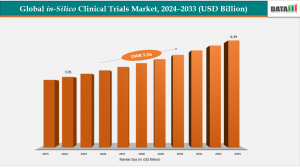
According to DataM Intelligence research report 2025. in-silico clinical trials market was valued at USD 3.76 billion in 2023 and increased to USD 3.95 billion in 2024. It is projected to reach USD 6.39 billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025–2033.. In-silico trials utilize computer simulations and modeling to predict drug efficacy and safety, reducing costs and accelerating drug development.
Download PDF Brochure: https://www.datamintelligence.com/download-sample/in-silico-clinical-trials-market
Recent Clinical Trials
1. In-silico cohorts and virtual patients increasingly supplement small Phase I or proof-of-concept studies, improving trial design, patient stratification, and early dose selection.
2. AstraZeneca utilized Quantitative Systems Pharmacology (QSP) models and virtual patients to accelerate PCSK9 therapy development, enabling a 6-month head start to Phase 2 trials.
3. Insilico Medicine initiated global multicenter Phase 1 dosing for its AI-designed cancer drug ISM3412, progressing rapidly leveraging in-silico validation of safety and pharmacokinetics.
4. Computational fluid dynamics (CFD) virtual trials predicted aneurysm flow reduction for Medtronic’s Pipeline Embolization Device, correlating well with real clinical trial outcomes.
Key Highlights from This Report
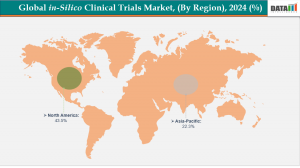
. North America led the in-silico clinical trials market in 2024, accounting for the highest revenue share at 43.5%.
. The Asia Pacific region is the fastest-growing market, projected to record the highest CAGR of 7.4% during the forecast period.
. Pharmacokinetic and pharmacodynamic (PK/PD) models dominated the market by model type, representing a 39.3% share in 2024.
. The drug development segment held the largest share by application, capturing 41.3% of the market in 2024.
Key Growth Drivers
Rising R&D costs and long timelines of traditional clinical trials.
Increasing adoption of AI, machine learning, and computational biology in pharma.
Regulatory encouragement of model-informed drug development approaches.
Growing need to reduce animal testing and ethical concerns.
Expansion of digital health platforms enabling data integration.
Market Segmentation Analysis
By Technology:
Computational modeling and simulation software lead the market.
AI/ML-driven analytics and predictive modeling are rapidly expanding.
By Application:
Drug discovery and efficacy testing dominate, with growing use in toxicology and dosage optimization.
By End User:
Pharmaceutical companies and Contract Research Organizations (CROs) are primary users.
Regulatory bodies and academic institutions contribute to development and validation.
Request for Customized Sample Report as per Your Business Requirement: https://www.datamintelligence.com/customize/in-silico-clinical-trials-market
Latest FDA Approvals and Drug Approvals
1. FDA has broadened guidance accepting computational modeling and verified virtual evidence to support device 510(k) submissions and biologic IND filings, reflecting regulatory endorsement of in-silico data.
2. Insilico Medicine received FDA IND clearance for ISM5939, an AI-designed oral small molecule targeting solid tumors, marking a landmark drug approval leveraging in-silico design.
3. FDA accepted in-silico PK/PD simulation data from Pfizer for tofacitinib formulations, reducing need for redundant phase 3 clinical trials report
Regional Insights
North America:
Largest market valued at USD 220 million in 2024, driven by strong pharma presence and technological leadership.
Europe:
Increasing investments in computational biology and clinical trial modernization initiatives.
Asia-Pacific:
Emerging market with growing clinical research outsourcing and digital transformation.
Key Players
Key companies include Certara || Dassault Systèmes || InSilicoTrials Technologies || Nova || Insilico Medicine || The AnyLogic Company || Simulations Plus || VeriSIM Life || Physiomics Plc, and ANSYS, Inc. Firms focus on enhancing simulation accuracy, expanding platform capabilities, and regulatory engagement.
Recent Developments
In May 2025, the FDA outlined a major transition away from animal testing, citing that more than 90% of drugs that succeed in animal studies fail in human trials. The updated roadmap prioritizes new approach methodologies, including human-based in vitro systems, in-silico modeling, and advanced platforms for evaluating immunogenicity, toxicity, and pharmacodynamic responses.
Certara’s new in-silico trial platform launched for oncology drug modeling (Q2 2025).
Partnerships between pharma and software developers to integrate real-world data.
Regulatory agencies issuing new guidelines supporting in-silico evidence submission.
Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer): https://www.datamintelligence.com/buy-now-page?report=in-silico-clinical-trials-market
Recent Procurements and Market Movement
1. Tempus AI acquired Deep 6 AI in March 2025, integrating real-time EMR analytics with AI-driven virtual patient matching to accelerate clinical trial recruitment.
2. CROs expanded in-silico trial services, with Worldwide Clinical Trials partnering with Medidata to combine eSource capture and virtual-patient simulation, increasing trial speed and reducing cost.
3. Investments in AI-driven drug discovery platforms including Absci, Owkin, and Recursion raised hundreds of millions, boosting computational modeling for preclinical and clinical workflows.
Market Outlook
In-silico clinical trials will significantly disrupt traditional drug development by lowering costs, improving prediction accuracy, and enabling personalized medicine, with adoption accelerating worldwide through 2032.
Related Reports
Clinical Trials Market Reaches $143B by 2033 - DataM Intelligence @ https://www.datamintelligence.com/research-report/clinical-trials-market
AI in Clinical Trials Market Growing to $5B by 2033 @ https://www.datamintelligence.com/research-report/ai-in-clinical-trials-market
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.